Zooming into biological structure
Building a mosaic of tools to see across spatial scales.
It is settled science that the brain is made up of billions of individual cells, the neurons. But well into the early 1900s, many scientists believed the brain was a continuous web of fibers rather than a collection of distinct cells.
Two leading figures stood on opposite sides of this question. Santiago Ramón y Cajal, a neuroanatomist and artist, drew what he saw under the microscope and argued that neurons were discrete units. Camillo Golgi, whose staining method made such drawings possible, believed the nervous system was one seamless network. In 1906, they shared the Nobel Prize in physiology, despite holding opposing views. The disagreement was like trying to decide whether a chain-link fence is made of separate pieces or one continuous sheet when you are only allowed a blurry photograph.
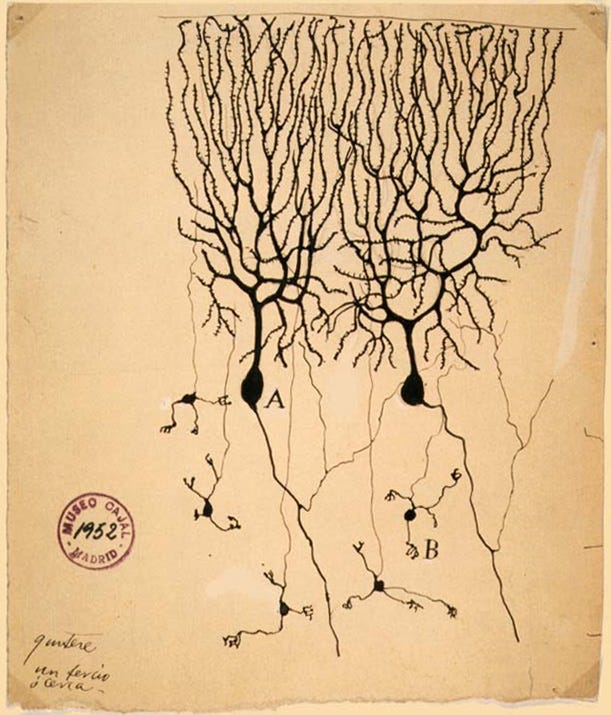
The connection point between neurons, what we now call the synapse, was invisible with the microscopes of their time. To Cajal and Golgi, the brain tissue under the microscope looked like a mesh, and the idea of a gap between cells remained purely theoretical. It would take another fifty years and major advances in imaging before scientists could directly see the synapse. Resolving this debate and embracing the concept of the synapse paved the way for understanding brain signaling and developing treatments for depression, epilepsy, and Parkinson’s disease.
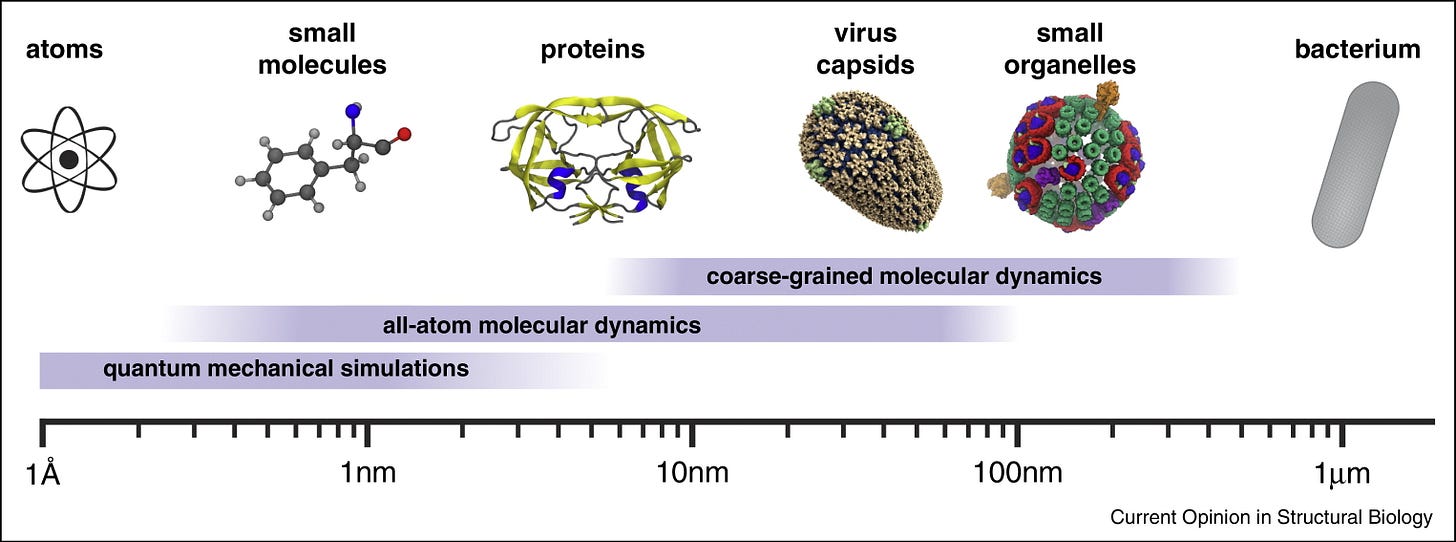
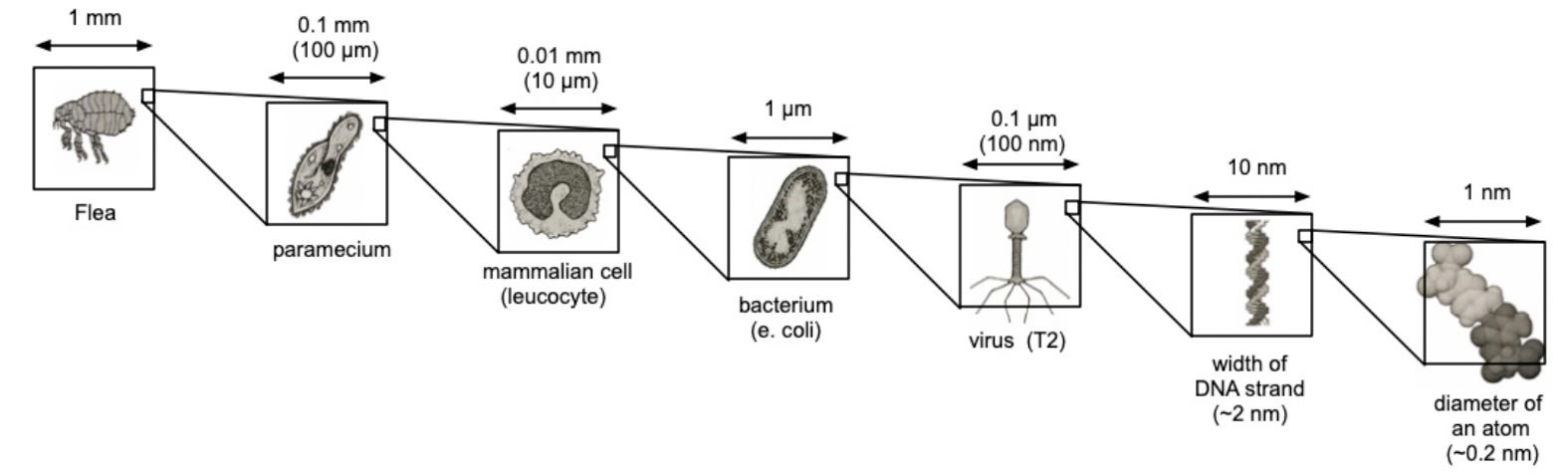
Studying biology has always depended on our ability to see structure in finer and finer detail. Life spans an enormous range of scales, from nanometers to centimeters. At the large end are whole organisms, from single-celled bacteria to complex mammals. At the small end are proteins and even the individual amino acids that make them up. In between lie viruses, organelles (the structures within cells), and the molecular machinery that keeps cells running and allows them to communicate with one another.
Understanding biological structure in high-resolution detail allows us to better predict function, design therapeutics, and develop vaccines for diseases. In 1953, X-ray crystallography revealed the structure of DNA’s double helix, laying the foundation for molecular genetics and modern biotechnology. In 1969, Dorothy Hodgkin’s crystal structure of the insulin molecule not only showed how insulin is stored in the body, but also guided the rational design of insulin analogs that are still central to diabetes treatment today. More recently, cryo-electron microscopy has become an essential tool in drug discovery and informing the development of improved antibody-based therapies.
There is no one-size-fits-all instrument that can image the large and the small. Instead, the field has evolved a multitude of approaches to study structure at different scales. We have traveled the spatial scale of millimeters, micrometers, nanometers, angstroms, and are now knocking on the door of the sub-angstrom world. Getting to this point took centuries of work.
Light Microscopy
From the 17th to the 19th century, light microscopy was biology’s only window into the fine structures of life. The earliest compound microscopes, like Galileo’s in the early 1600s, offered just 30 times magnification, which was enough to see insect legs, hairs, and the outlines of large plant cells, but still blind to red blood cells or bacteria.
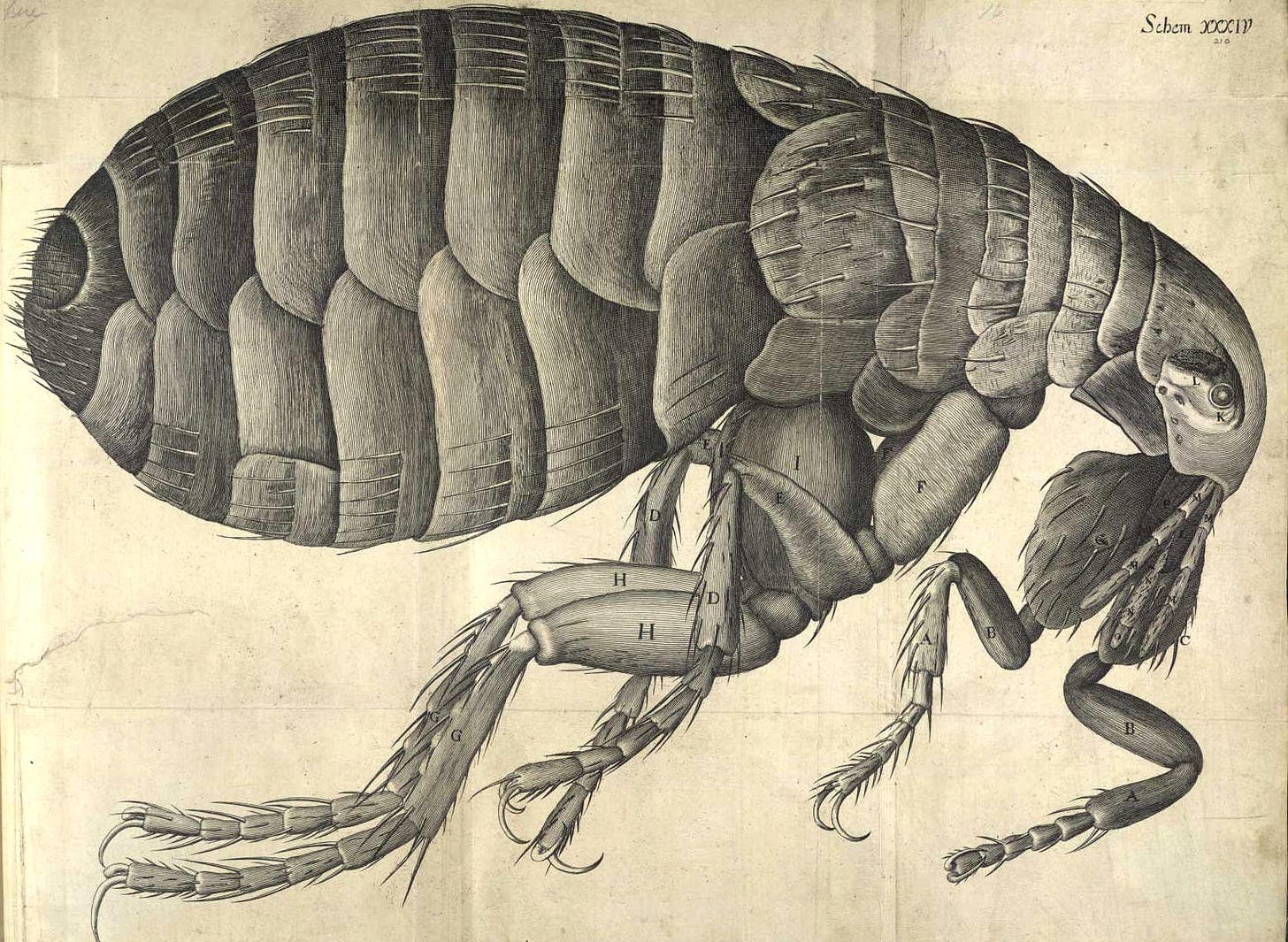
That leap came from Antonie van Leeuwenhoek, a Dutch microbiologist and microscopist who turned microscopy into an art form. By the late 1600s, his meticulously crafted single-lens microscopes reached 300 times magnification with resolutions down to one micrometer. For the first time, blood cells, bacteria, and protozoa came into view.
But this microscopic world existed only in memory and sketches of its observers. For centuries, scientists had no way to capture what they saw beyond hand drawings. It was only in the late 1800s, after the invention of photography, that scientists first began experimenting with photographing microscopic observations.
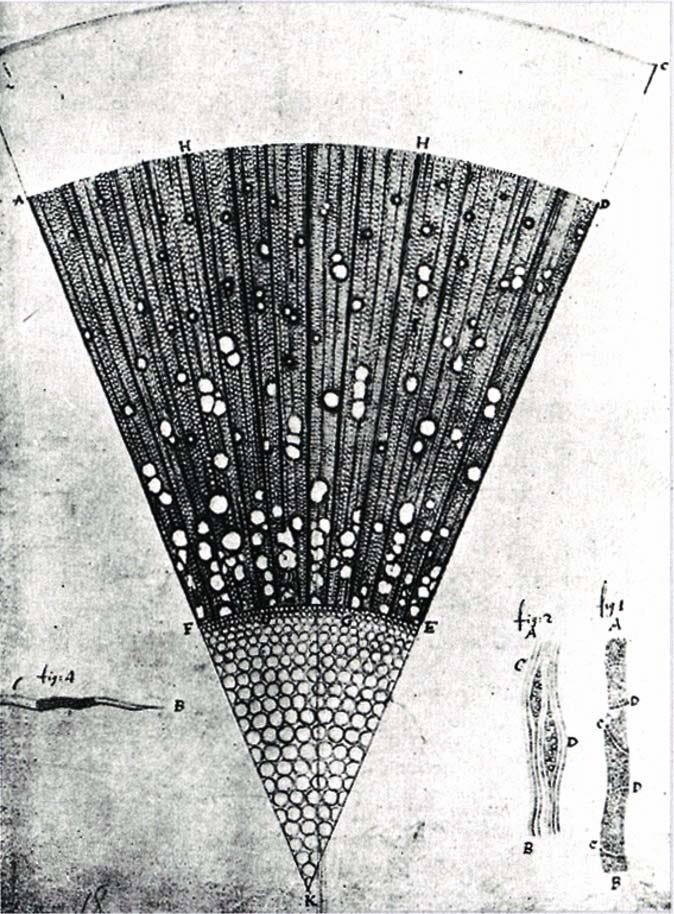
Meanwhile, van Leeuwenhoek’s single-lens microscopes had a problem: a tiny field of view. Early compound microscopes with two or more lenses promised to fix this, but the added optics created severe color aberration, which created a rainbow halo around every specimen because different wavelengths bent at different angles. In the mid-1700s, lens makers solved this by combining different glass types into the achromatic doublet, which canceled out the color fringing.
By the early 1800s, achromatic lenses gave compound microscopes the resolution of van Leeuwenhoek’s instruments but with wider fields of view. Scientists could now image cells within whole tissues, like plant stems, animal organs, the kinds of specimens familiar to any high school biology student today. This clarity fueled the cell theory in 1839, establishing that all living organisms are made of cells and that the cell is life’s fundamental unit.
Then came the precision era. In the late 1800s, Carl Zeiss and Ernst Abbe transformed microscopy from artisanal tinkering into optical engineering. Using mathematical optics, they designed lenses systematically, pushing resolution to about 0.25 micrometers. Now, cell membranes, nuclei, mitochondria, and bacterial shapes all came into sharp focus.
By this time, the light microscope was reaching its limits. In 1873, Abbe mathematically proved that no matter how perfect the lenses, light microscopes could not resolve details smaller than ~200 nanometers, which is about 250 times thinner than a human hair. Much of biology, including synapses, viruses, and proteins, lies hidden below this line. The next leaps in imaging would require breaking the limits of light itself.
Into the Invisible
In 1895, X-rays were discovered. As physicists worked to understand their nature, they found that X-rays behaved like waves, and that crystals acted like 3D diffraction gratings when X-rays passed through them. In 1913, William and Lawrence Bragg, the father–son duo, realized they could use these diffraction patterns to deduce crystal structures mathematically, inventing X-ray crystallography. In X-ray crystallography, X-rays scatter through a crystal to produce a pattern of spots on a screen. Analyzing those patterns reveals the arrangement of atoms inside the crystal down to 0.3 nanometers in the best cases.
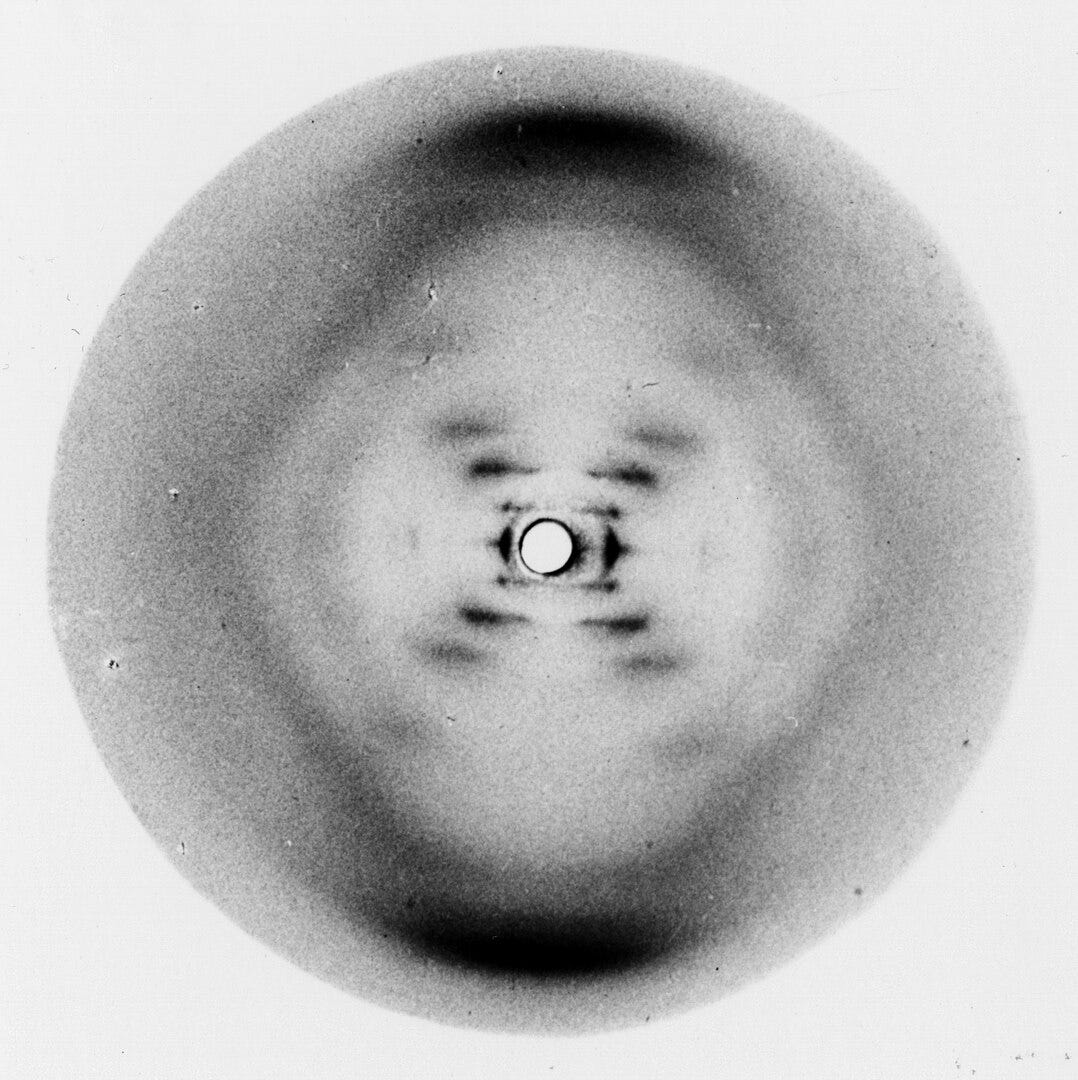
Soon, this powerful technique was applied to biology. In 1934, Dorothy Hodgkin and J.D. Bernal demonstrated that protein crystals, if carefully prepared, could also yield X-ray diffraction patterns, leading to the birth of structural biology. The next few decades delivered landmark discoveries: the double helix of DNA in 1953, the first protein structures (myoglobin and hemoglobin) in the late 1950s. Biology moved from describing life’s parts to understanding them as 3D machines whose shapes explained their functions.
But X-ray crystallography had limits. It required crystals, ruling out most large proteins, viruses, and cellular assemblies. And because no lens could bend X-rays, scientists only saw diffraction patterns, never direct images. The vast middle ground between small crystallizable molecules and whole cells imaged by light microscopy remained invisible.
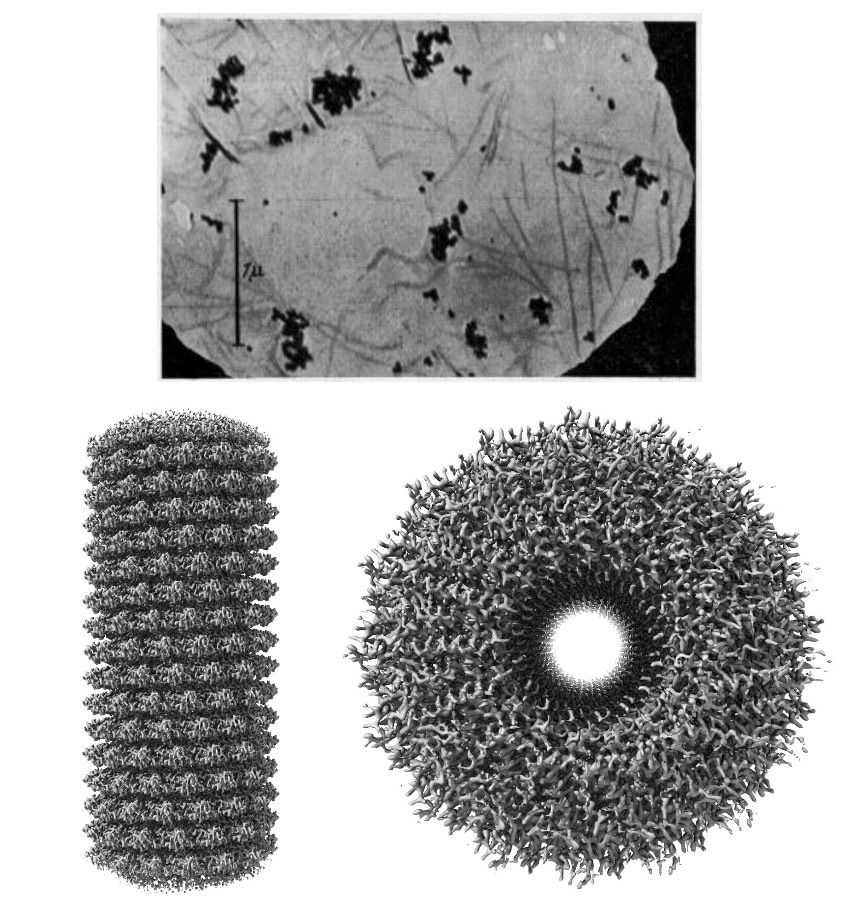
The electron microscope filled that gap. Invented by Ernst Ruska in 1931, it used electron beams, with wavelengths far shorter than light, to achieve much higher resolution. By the late 1930s, researchers could see entire viruses like tobacco mosaic virus and vaccinia for the first time, proving these “germs” were real physical particles and validating the germ theory of disease. Early electron micrographs also revealed mitochondria, nuclei, and the internal architecture of cells’ structures that had been guessed at but never seen.
Over the next decades, electron microscopy kept advancing. New staining and sample preparation methods in the 1950s uncovered membranes and organelles in unprecedented detail. The 1960s brought scanning electron microscopy, producing dramatic 3D-like views of cell surfaces. Then came the revolution of cryogenic electron microscopy (cryo-EM) in the 1980s and 1990s, where samples were rapidly frozen, preserving them close to their native state. Today, cryo-EM can reach near-atomic resolution for proteins, viruses, and organelles, finally linking the atomic details revealed by X-ray crystallography to the cellular landscapes seen with light microscopy.
From Images to Insights
Over the past four centuries, biology has advanced hand-in-hand with the instruments used to visualize it. From the early single-lens microscopes that revealed blood cells and bacteria, to X-ray diffraction patterns that uncovered the atomic scaffolding of DNA and proteins, and then to electron microscopes that brought viruses and cell ultrastructure into view, each leap in imaging has opened a new layer of the biological world. Together, these approaches form a mosaic that spans scales from nanometers to centimeters, connecting the structure of molecules to the organization of tissues.
This is far from an exhaustive list. Even as electron microscopy and X-ray crystallography transformed biology, light microscopy never stood still. Techniques like confocal microscopy, light-sheet microscopy, super-resolution methods, and expansion microscopy have pushed past earlier limits to image cells in ever greater detail. Other approaches, such as nuclear magnetic resonance (NMR) and single-molecule spectroscopy, opened additional windows onto molecular structure and dynamics. Rather than replacing one another, these techniques have filled complementary niches, each expanding what could be visualized in space or time.
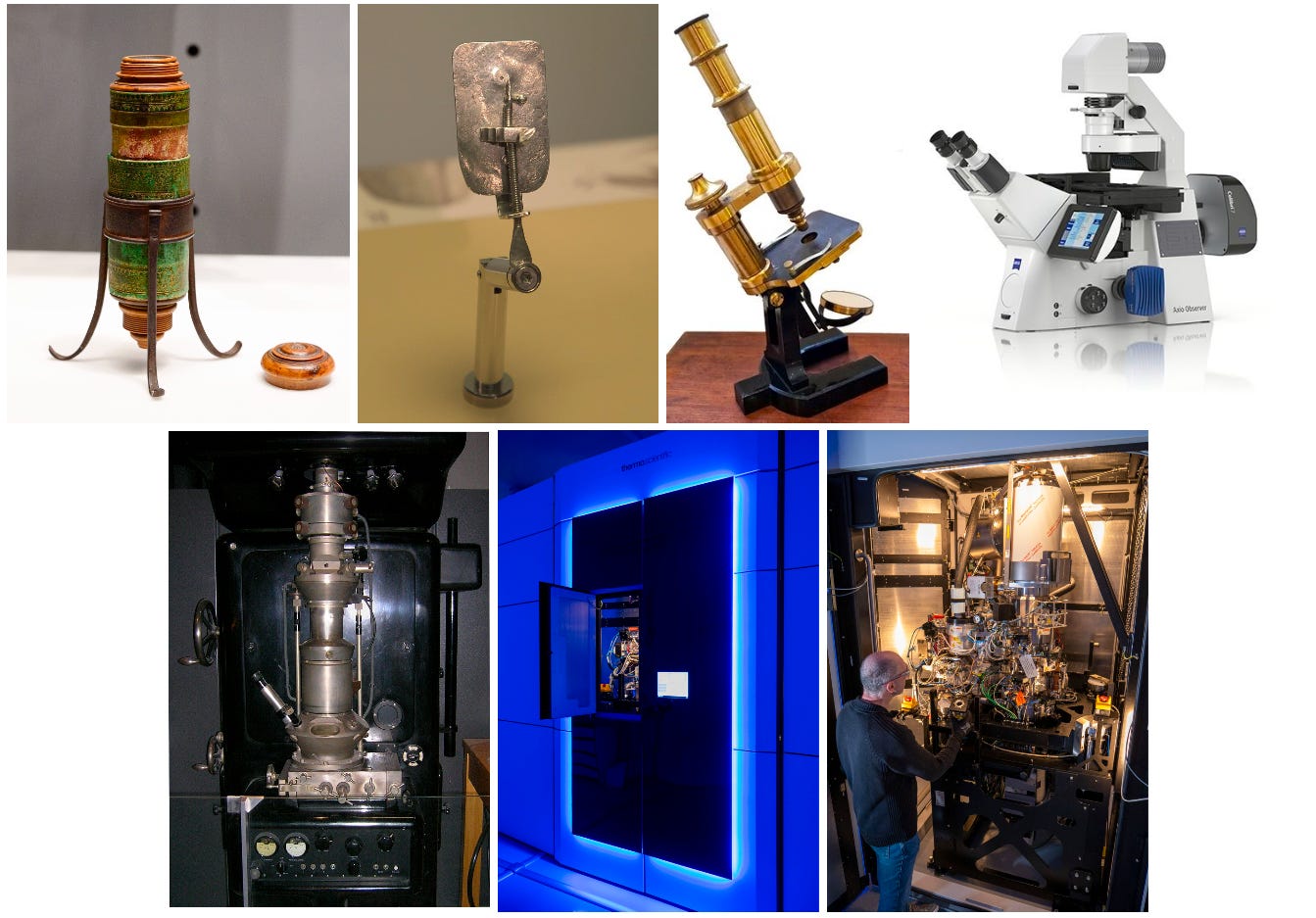
This essay has focused on static structure, which is the ability to freeze life at different scales and examine it in detail. An equally important frontier has been dynamic imaging: light microscopes adapted to look inside living cells, fluorescent proteins that allow individual molecules to be tracked in real time, and super-resolution methods that extend beyond the diffraction limit of light. Structural and dynamic approaches together provide a fuller picture of biology. Our ability to see into biology is inseparable from our ability to understand, and each improvement in technology reshapes the questions that we could ask about biology.


Very interesting essay, Smrithi. I wish our kids learned more about this "how we know" in school. Alas, most of the time they just learn what we know, but not how. I wonder if there is an essay you (or someone!) could write about the how we know for specific things that high school students need to learn in biology. Mitochondria, ATP, the Krebs cycle--all the stuff that teens memorize and parrot without understanding: how much more interesting it would be if these things were taught as detective stories, with the machinery of knowing that you describe here as part of how we got clues to our modern understanding.